Main Article Content
Abstract
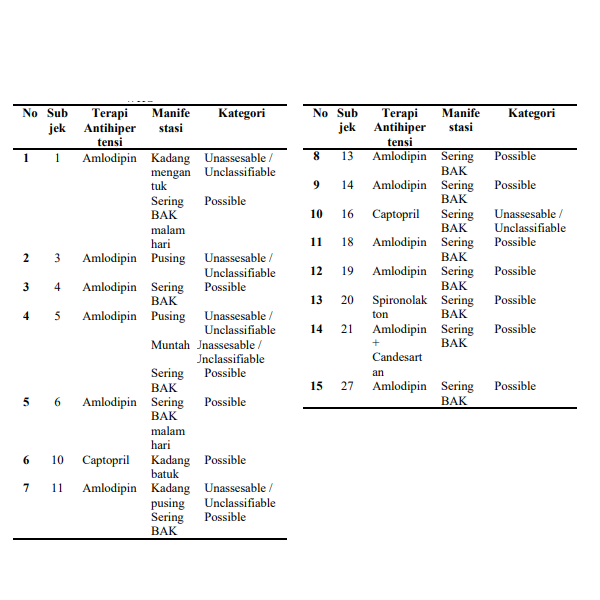
Indonesia is a country with a fairly low level of health awareness, the number of patients who do not realize that they are suffering from hypertension and who do not use drugs rationally, causing side effects is quite high. Several publications show that antihypertensive drug therapy triggers a high incidence of ROTD in society. The purpose of this study was to determine the presence or absence of ADR in the treatment of hypertension with synthetic anti hypertension drugs using the WHO-UMC method. This study used a cross-sectional study method which is a form of observational study (non-experimental). The results obtained in this study were 15 people (50%) who reported the absence of ROTD and 15 people (50%) reported the absence of ROTD. In this study, it is known that there is ROTD with the use of the drugs amlodipine, captopril, spironolactone and a combination of amlodipine with candesartan. ROTD that occurred in 11 subjects was classified as possible, which means that there is a possibility that the drug causes ROTD in the form of an increase in the frequency of BAK. The conclusion of this study is that there is a synthetic antihypertensive drug therapy that found the incidence of ROTD in the form of an increase in the frequency of BAK in the use of captopril drugs and the incidence of dizziness, vomiting, and drowsiness on the use of the drug amlodipine.
Keywords
Farmakovigilans
Antihipertensi
Hipertensi
ROTD
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Yuwindry, I., Agustina, A., & Kurniawati, D. (2021). Studi pharmacovigilance obat antihipertensi sintetis pada pasien hStudi Pharmacovigilance Obat Antihipertensi Sintetis pada Pasien Hipertensi di Kota Banjarmasinipertensi di kota Banjarmasin. Borneo Journal of Pharmascientech, 5(2), 55-63. https://doi.org/10.51817/bjp.v5i2.305